SH2 Domain-Containing Phosphatase 2 Inhibition Attenuates Osteoarthritis byMaintaining Homeostasis of Cartilage Metabolism via the Docking Protein1/Uridine Phosphorylase 1/Uridine Cascade.
Liu Q, Zhai L, Han M, Shi D, Sun Z, Peng S, Wang M, ZhangC, Gao J, Yan W, Jiang Q, Chen D, Xu Q, Tan M, Sun Y.
Arthritis Rheumatol. 2022 Mar;74(3):462-474. doi: 10.1002/art.41988.
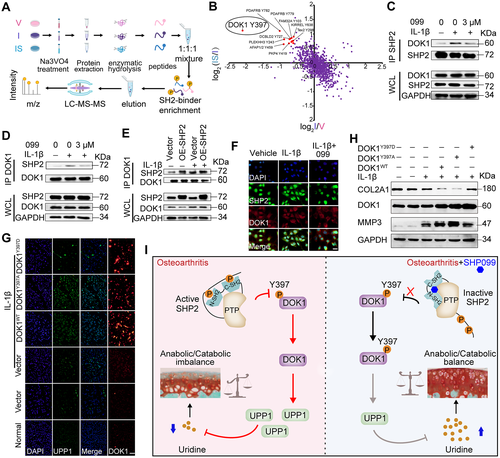
OBJECTIVE: Protein tyrosine kinases regulate osteoarthritis (OA) progression byactivating a series of signal transduction pathways. However, the roles ofprotein tyrosine phosphatases (PTPs) in OA remain obscure. This study wasundertaken to identify specific PTPs involved in OA and investigate theirunderlying mechanisms.METHODS: The expression of 107 PTP genes in human OA cartilage was analyzedbased on a single-cell sequencing data set. The enzyme activity of the PTP SH2domain-containing phosphatase 2 (SHP-2) was detected in primary chondrocytesafter interleukin-1尾 (IL-1尾) treatment and in human OA cartilage. Mice subjectedto destabilization of the medial meniscus (DMM) and IL-1尾-stimulated mouseprimary chondrocytes were treated with an SHP-2 inhibitor or celecoxib (a drugused for the clinical treatment of OA). The function of SHP-2 in OA pathogenesiswas further verified in Aggrecan-CreERT ;SHP2flox/flox mice. The downstreamprotein expression profile and dephosphorylated substrate of SHP-2 were examinedby tandem mass tag labeling-based global proteomic analysis and stable isotopelabeling with amino acids in cell culture-labeled tyrosine phosphoproteomicanalysis, respectively.RESULTS: SHP-2 enzyme activity significantly increased in human OA samples withserious articular cartilage injury and in IL-1尾-stimulated mouse chondrocytes.Pharmacologic inhibition or genetic deletion of SHP-2 ameliorated OAprogression. SHP-2 inhibitors dramatically reduced the expression of cartilagedegradation-related genes and simultaneously promoted the expression ofcartilage synthesis-related genes. Mechanistically, SHP-2 inhibition suppressedthe dephosphorylation of docking protein 1 and subsequently reduced theexpression of uridine phosphorylase 1 and increased the uridine level, therebycontributing to the homeostasis of cartilage metabolism.CONCLUSION: SHP-2 is a novel accelerator of the imbalance in cartilagehomeostasis. Specific inhibition of SHP-2 may ameliorate OA by maintaining theanabolic-catabolic balance.